Abstract
Background: Acute promyelocytic leukemia (APL) is a highly curable subtype of Acute myelogenous leukemia (AML). The groundbreaking APL 0406 trial, Lo-Coco et al, showed improved CR and OR with Al-trans retinoic acid (ATRA) + Arsenic trioxide (ATO) compared to ATRA + chemotherapy in patients with standard-risk APL. Although clinical trials have demonstrated survival rates of >90%, induction complications and mortality continue to be a significant problem with some national registries reporting induction deaths as high as 20-30% due to infections, bleeding, disseminated intravascular coagulation DIC, and/or differentiation syndrome DS. In this abstract we evaluate an underreported and underdiagnosed acute musculoskeletal (MSK) pain syndrome in a subset of APL patients receiving induction therapy.
Methods: Chart review of patients with confirmed APL who underwent induction therapy at our leukemia center from 03/2014 to 07/2022 were included in this study. A total of 42 patients were screened for a reported episode of an "acute musculoskeletal pain event” following the initiation of induction therapy. Acute musculoskeletal pain event was defined as an MSK pain involving lower and/or upper extremities and/or rib cage and/or pelvic and/or chest with a pain score intensity of >6, followed by exclusion of any other etiology of pain, and requiring some form of medical intervention. Patients were also screened for DS using PETHMA DS severity gradation (severe >= 4, moderate 3, indeterminate <=2 features) to see if there was any association with MSK pain events and DS.Institutional review board approval was obtained for this review.
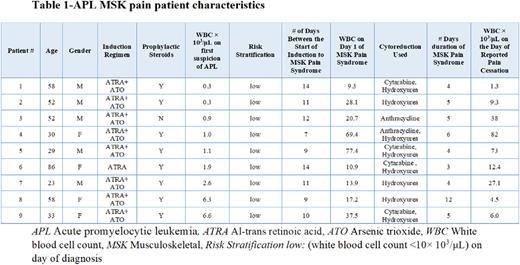
Results: n=42 patients were induced from 03/2014 to 07/2022. (n=9, 21.4%) met the criteria for an acute MSK pain event, median age was 52 years ranging from 23-86, (n=5, 55.6%) were male. The predominant induction combination was ATRA + ATO (n=8, 88.9%), whereas (n=1 ,11.1%) received ATRA alone due to older age. All 9 patients were stratified as low-risk (white blood cell count <10× 103/μL) with a median WBC of 1.1× 103/μL ranging from 0.3-6.6 × 103/μL on the day of first suspicion of APL. The median time to presentation of the acute MSK pain event from the start of induction therapy was 11 days ranging from 7-14 days. Median WBC count on the first day of the reported pain event was 20.7 × 103/μL with a range of 9.3-69.4× 103/μL. The median duration of the MSK pain event was 5 days ranging from 4-12 days with a reported median WBC of 12.4 × 103/μL and range of 1.3-82 × 103/μL on the day of pain cessation. (n=7, 77.8%) patients had their ATRA interrupted, (n=5, 55.6%) had ATO interrupted and (n=5, 55.6%) had both ATRA/ATO interrupted temporarily during the induction period due to worsening pain and increasing WBC count. (n=8, 88.9%) received hydroxyurea and in addition (n=2, 22.2%) patients received anthracyclines, and (n=4, 44.4%) received cytarabine for immediate cytoreduction. None of the patients had reported DS, median PETHEMA DS severity gradient score was 1 (indeterminate <=2 features) with the scores ranging from 0-2. No mortalities occurred during the induction period.
Conclusions: All 9 patients experiencing our reported MSK pain syndrome were stratified as low-risk. None of the high-risk patients (n=12, 29%) met criteria for acute MSK pain syndrome which may be a result of the administration of cytotoxic chemotherapy early in the course of the disease. This suggests that the pain syndrome is mostly seen during a rapid rise in WBC following the start of induction therapy in low-risk patients. None of the 9 patients met diagnostic criteria for DS. Further attention should be paid to this phenomenon to better understand its pathophysiology and implications during the APL induction period.
Disclosures
Kolhe:Perkin Elmer: Honoraria, Research Funding; PGDx: Honoraria, Research Funding; Illumina: Research Funding; Bioanano INc: Honoraria, Research Funding, Speakers Bureau; Agena: Honoraria, Research Funding; Qiagen: Honoraria, Research Funding; Cepheid: Honoraria. Kota:Xcenda: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Ariad: Honoraria; Pfizer Inc: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal